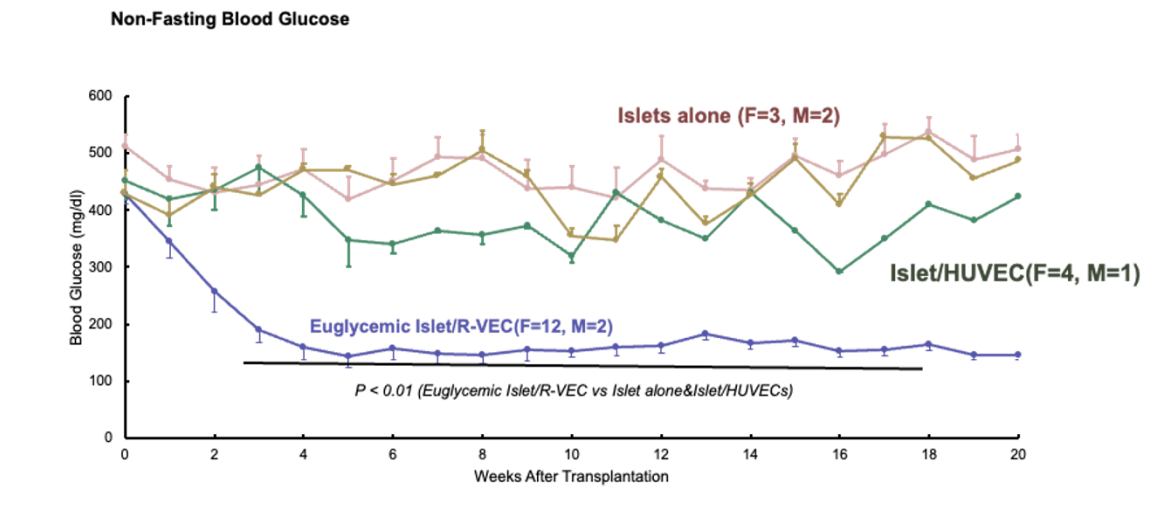
Organoid-based Blood Glucose Control In Kidney
Transplant
Recipients With Type 1 Diabetes
 Diabetes is leading cause of
kidney disease in the US. 1 of 3 on the kidney transplant waiting list has diabetic
end-stage renal disease
Diabetes is leading cause of
kidney disease in the US. 1 of 3 on the kidney transplant waiting list has diabetic
end-stage renal disease Type-1 diabetics see the worst
outcomes post-transplant, with survival dropping to half that of non-diabetics in 15
years
Type-1 diabetics see the worst
outcomes post-transplant, with survival dropping to half that of non-diabetics in 15
years Kidney transplant
recipients are
already treated with chronic immunosuppressives
Kidney transplant
recipients are
already treated with chronic immunosuppressives That treatment would also
prevent
rejection of donor islets used with R-VECs
That treatment would also
prevent
rejection of donor islets used with R-VECs •PRO-303, injected
subcutaneously, would provide long-lasting blood glucose control to preserve donor
kidney function and avoid T1D-related complications
•PRO-303, injected
subcutaneously, would provide long-lasting blood glucose control to preserve donor
kidney function and avoid T1D-related complications